- 抑制劑
- 化合物庫
- 抗體
- 生物試劑
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- 蛋白實驗
- Protein A/G免疫沉淀磁珠
- Anti-DYKDDDDK Tag免疫磁珠
- Anti-DYKDDDDK Tag親和凝膠
- Anti-Myc免疫磁珠
- Anti-HA免疫磁珠
- 磁力架
- Poly DYKDDDDK Tag多肽
- 細(xì)胞核與細(xì)胞漿蛋白抽提試劑盒
- 免疫磁珠
- 蛋白酶抑制劑Cocktail
- 蛋白酶抑制劑Cocktail(DMSO儲液)
- 磷酸酶抑制劑Cocktail
- 細(xì)胞實驗
- Cell Counting Kit-8 (CCK-8)
- 新產(chǎn)品
- 聯(lián)系我們
Asunaprevir
別名: BMS-650032 中文名稱:阿那匹韋
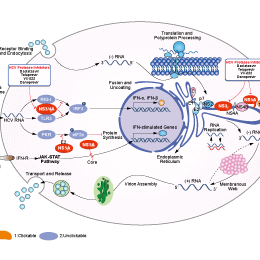
Asunaprevir是具有口服活性的HCV NS3抑制劑。HCV NS3是病毒復(fù)制蛋白加工所必需的蛋白酶。
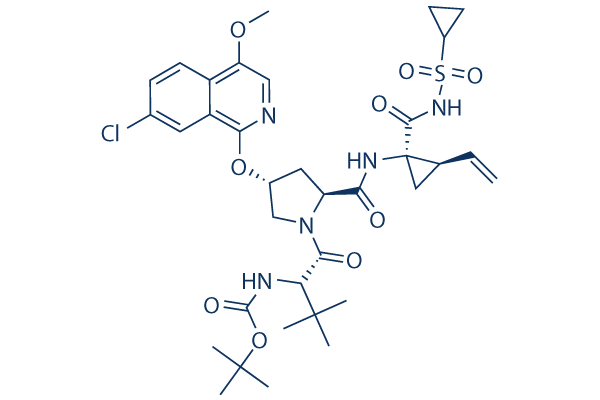
Asunaprevir Chemical Structure
CAS: 630420-16-5


產(chǎn)品質(zhì)控
Asunaprevir相關(guān)產(chǎn)品
| 相關(guān)產(chǎn)品 | Lomibuvir (VX-222) Danoprevir PSI-6206 (GS-331007) | 點擊展開 |
|---|---|---|
| 相關(guān)化合物庫 | FDA藥物庫 天然產(chǎn)物庫 已知活性藥物庫-I 蛋白酶抑制劑庫 泛素化化合物庫 | 點擊展開 |
相關(guān)信號通路圖
細(xì)胞實驗數(shù)據(jù)示例
| 細(xì)胞系 | 實驗類型 | 給藥濃度 | 孵育時間 | 活性描述 | 文獻(xiàn)信息(PMID) |
|---|---|---|---|---|---|
| Huh7.5 replicon cells | Antiviral assay | 4 days | Antiviral activity against HCV genotype 1b infected in human Huh7.5 replicon cells assessed as reduction in viral replication incubated for 4 days by luciferase reporter gene assay, EC50=0.006μM | 29650290 | |
| HuH7.5 cells | Antiviral assay | 4 days | Antiviral activity against HCV genotype 1b Con1 infected in human HuH7.5 cells assessed as inhibition of viral replication after 4 days by luciferase reporter gene assay, EC50=0.006μM | 29456803 | |
| HuH7 replicon cells | Function assay | 4 days | Inhibition of HCV genotype 1a H77 NS3 protease infected in human HuH7 replicon cells assessed as reduction in viral replication after 4 days by luciferase reporter gene assay, EC50=0.004μM | 29162454 | |
| HuH7 replicon cells | Function assay | 4 days | Inhibition of HCV genotype 1b Con1 NS3 protease infected in human HuH7 replicon cells assessed as reduction in viral replication after 4 days by luciferase reporter gene assay, EC50=0.0012μM | 29162454 | |
| HuH7 cells | Antiviral assay | 3 days | Antiviral activity against HCV genotype 1b infected in human HuH7 cells co-treated with daclatasvir after 3 days by luciferase reporter assay | 28430437 | |
| HuH7 cells | Antiviral assay | 3 days | Antiviral activity against HCV genotype 1b infected in human HuH7 cells at >= 10 times antiviral EC50 after 3 days by luciferase reporter assay | 28430437 | |
| genotype 2a replicon cells | Function assay | Inhibition of recombinant full length HCV genotype 3a S52 NS3 protease in genotype 2a replicon cells by luciferase reporter gene assay, EC50=1.1μM | 27564532 | ||
| genotype 2a replicon cells | Function assay | Inhibition of recombinant full length HCV genotype 2b HC-J8 NS3 protease in genotype 2a replicon cells by luciferase reporter gene assay, EC50=0.621μM | 27564532 | ||
| HCV replicon cells | Antiviral assay | Antiviral activity against HCV genotype 2a JHF-1 in HCV replicon cells assessed as reduction in viral RNA replication by luciferase reporter gene assay, EC50=0.217μM | 27564532 | ||
| HCV replicon cells | Antiviral assay | Antiviral activity against HCV genotype 1a H77 in HCV replicon cells assessed as reduction in viral RNA replication by luciferase reporter gene assay, EC50=0.004μM | 27564532 | ||
| Huh7.5 cells | Antiviral assay | Antiviral activity against HCV genotype 1b Con1 expressing NS3 protease infected in human Huh7.5 cells assessed as reduction in viral RNA replication by luciferase reporter gene assay, EC50=0.003μM | 27564532 | ||
| genotype 1b con1 replicon cells | Function assay | Inhibition of recombinant full length HCV genotype 4a ED43 NS3 protease in genotype 1b con1 replicon cells by luciferase reporter gene assay, EC50=0.0017μM | 27564532 | ||
| 點擊查看更多細(xì)胞系數(shù)據(jù) | |||||
生物活性
| 產(chǎn)品描述 | Asunaprevir是具有口服活性的HCV NS3抑制劑。HCV NS3是病毒復(fù)制蛋白加工所必需的蛋白酶。 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 靶點 |
|
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03208322 | Withdrawn | Hepatitis C |
Bristol-Myers Squibb |
November 30 2018 | -- |
| NCT03004625 | Completed | Hepatitis C |
Kaohsiung Medical University Chung-Ho Memorial Hospital|Chang Gung Memorial Hospital|National Taiwan University Hospital|Taipei Veterans General Hospital Taiwan|China Medical University Hospital|National Cheng-Kung University Hospital |
November 2016 | Phase 3 |
| NCT02865369 | Unknown status | Chronic Hepatitis C |
Sang Gyune Kim|Seoul National University Boramae Hospital|Severance Hospital|Inha University Hospital|Korea University|Gachon University Gil Medical Center|Hanyang University Seoul Hospital|Ewha Womans University Mokdong Hospital|Bristol-Myers Squibb|Soonchunhyang University Hospital |
September 2016 | -- |
| NCT02580474 | Completed | Hepatitis C |
Myeong Jun Song|Bristol-Myers Squibb|Soonchunhyang University Hospital|Dankook University|Chungnam National University Hospital|Konyang University Hospital|Eulji University Hospital|Saint Vincent''s Hospital Korea|Konkuk University Hospital|Cheongju St. Mary''s Hospital Cheongju Korea|Severance Hospital|Korea University Guro Hospital|Eulji General Hospital|The Catholic University of Korea |
February 2016 | Phase 4 |
| NCT02496078 | Completed | Hepatitis C |
Bristol-Myers Squibb |
August 2015 | Phase 3 |
| NCT02309450 | Withdrawn | Hepatitis C Virus Genotype 4 Infection |
ANRS Emerging Infectious Diseases|Bristol-Myers Squibb |
December 2014 | Phase 2 |
|
化學(xué)信息&溶解度
| 分子量 | 748.29 | 分子式 | C35H46ClN5O9S |
| CAS號 | 630420-16-5 | SDF | -- |
| Smiles | CC(C)(C)C(C(=O)N1CC(CC1C(=O)NC2(CC2C=C)C(=O)NS(=O)(=O)C3CC3)OC4=NC=C(C5=C4C=C(C=C5)Cl)OC)NC(=O)OC(C)(C)C | ||
| 儲存條件(自收到貨起) | |||
|
體外溶解度 |
DMSO : 100 mg/mL ( (133.63 mM) ;DMSO吸濕會降低化合物溶解度,請使用新開封DMSO) Ethanol : 100 mg/mL (133.63 mM) Water : Insoluble |
摩爾濃度計算器 |
|
體內(nèi)溶解配方 現(xiàn)配現(xiàn)用,請按從左到右的順序依次添加,澄清后再加入下一溶劑 |
動物體內(nèi)配方計算器 | |||||
實驗計算
動物體內(nèi)配方計算器(澄清溶液)
第一步:請輸入基本實驗信息(考慮到實驗過程中的損耗,建議多配一只動物的藥量)
第二步:請輸入動物體內(nèi)配方組成(配方適用于不溶于水的藥物;不同批次藥物配方比例不同,請聯(lián)系Selleck為您提供正確的澄清溶液配方)
計算結(jié)果:
工作液濃度: mg/ml;
DMSO母液配制方法: mg 藥物溶于μL DMSO溶液(母液濃度mg/mL,注:如該濃度超過該批次藥物DMSO溶解度,請先聯(lián)系Selleck);
體內(nèi)配方配制方法:取μL DMSO母液,加入μL PEG300,混勻澄清后加入μL Tween 80,混勻澄清后加入μL ddH2O,混勻澄清。
體內(nèi)配方配制方法:取μL DMSO母液,加入μL Corn oil,混勻澄清。
注意:1. 首先保證母液是澄清的;
2.一定要按照順序依次將溶劑加入,進(jìn)行下一步操作之前必須保證上一步操作得到的是澄清的溶液,可采用渦旋、超聲或水浴加熱等物理方法助溶。
技術(shù)支持
在訂購、運輸、儲存和使用我們的產(chǎn)品的任何階段,您遇到的任何問題,均可以通過撥打我們的熱線電話400-668-6834,或者技術(shù)支持郵箱[email protected],直接聯(lián)系到我們。我們會在24小時內(nèi)盡快聯(lián)系您。
如果有其他問題,請給我們留言。
* 必填項